Demystifying Battery Technology
This is a quick primer to key aspects of batteries for someone like me who knows a little about software but not very much about electrical engineering, power systems or embedding programming.
Introduction:¶
Batteries are key technological component in many electronic and electro-mechanical systems. But, I find myself thinking of them as substrate or a fuel tank, rather than a technology. Partly this is because of ignorance and partly by design. As a person making software * applications of batteries: smartphones to electric vehicles
Understanding Battery Basics¶
The term battery derives from a military battery. They are old, they were on of the predominant forms of electricity generation in say the 1840s although we don't generally think of them as generators today.
Batteries derive from physical chemistry, and they were a driving engine in the development of not just electrochemistry but the general theory of electro-magnetism. Michael Faraday coined the terms: - anode - cathode - electrode - ion
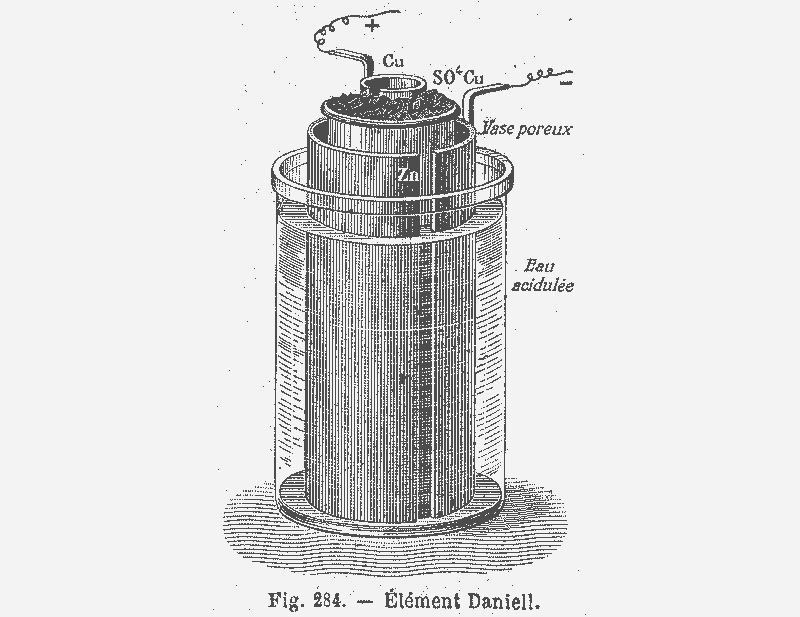
Introduction to galvanic cells.¶
Batteries are types of electro-chemical cells, that is chemical devices which do work either converting chemical energy into electrical or visa versa. Cells can be classified by the conversion type: galvanic cells convert chemical energy into electricity while electrolytic cells require electricity to perform a chemical reaction.
Both cell types are used in batteries: primary batteries like commercial alkaline AA driven by a reaction between zinc metal and manganese dioxide, are, as we know, not rechargeable. That's what makes a primary battery such, and they consist only of galvanic cells. Secondary batteries like the lithium-ion battery power my laptop are rechargeable, and the recharge process is the conversion of electrical energy from my ac adapter into chemical energy.
These are respective: - electrolyte - ions - separator - cell vs battery - open circuit voltage - internal resistance - capacity - c and c rate (capacity) - load - charging rate - discharge rate - state of charge - chemistry - primary (non-rechargeable) vs secondary (rechargeable) - cylinder vs prismatic vs pouch - battery refresh - charge time - chaging cycle or cycle life - use time - shelf life / service life - energy density - power density
- Types of batteries: an overview.
- Energy conversion in batteries.
- Components of batteries.
- Principle of operation.
Factors Affecting Battery Choice and Performance:¶
- Performance parameters to consider.
- Understanding battery voltage.
- Exploring secondary battery systems.
- Identifying battery-limiting factors.
- Effects of current and discharge modes.
- Impact of temperature on battery performance.
- Managing self-discharge.
- Considering calendar and cycle life.
- Understanding internal resistance and safety measures.
Deep Dive into Battery Types:¶
Although we briefly discuss other battery chemistries for context, lithium ion batteries are, rightly, the star of the show. The 2019 nobel prize in chemistry was awarded for the development of li-ion batteries.
- Lead-Acid Batteries:
- Nickel-Cadmium Batteries:
- Nickel-Metal Hydride Batteries:
- Lithium Batteries:
- Overview and evolution.
- Early, current and future lithium batteries: Li Metal; Li-Air and Li-S.; Li-Ion.
- Components and fabrication techniques.
- Charging and discharging processes.
- Extending cycle life and minimizing self-discharge.
- Addressing operational challenges.
- alternatives (supercapacitors, fuel cells, flow batteries, wireless power, solid state, etc.)
Battery Management Systems¶
- charging, charge controllers and charger techniques
- safety and protection
- cell-balancing
- battery fuel gauging